María José Gosalbes, Carlos D. Esteban and Gaspar Pérez-Martínez.
Published in Microbiology (2002) 148:695-702
Pubmed link: http://www.ncbi.nlm.nih.gov/pubmed/11882703. Full text:
SUMMARY:
The antiterminator LacT regulates the expression of the lactose operon in Lactobacillus casei and its activity is controlled by EIILac and common PTS elements. LacT shows the two conserved domains (PRD-I and PRD-II) characteristic of the BglG antiterminator family that are implicated in the regulation of their activity, possibly by phosphorylation of conserved histidines. By site-directed mutagenesis of LacT, four histidines (His-101, His-159 in PRD-I and His-210, His-273 in PRD-II) have been replaced by alanine or aspartate, mimicking non-phosphorylated and phosphorylated forms, respectively. These constructions were used to complement DlacT and DccpA mutants. L. casei strains (DlacT), carrying the replacement of His-101 or His-159 by Ala, showed P-b-galactosidase activity in absence of the inducer (lactose) indicating that these amino acids, located in PRD-I, are essential for EII-dependent induction of the lac operon, possibly by dephosphorylation. Interestingly, these mutations rendered LacT thermosensitive. Moreover, expression of H210A and H273A (PRD-II) mutations in L. casei DccpA showed that these two histidyl residues could have a role in LacT-dependent carbon catabolite repression (CCR) of this system. Overexpression of LacT in ccpA background rendered the lac operon insensitive to CCR, but it was still sensitive to lactose induction. Which suggest that the transfer of phosphate groups from PTS elements, that controls these two regulatory processes (CCR and substrate induction), could have different affinity for PRD-I and PRD-II histidines.
INTRODUCTION:
In lactic acid bacteria (LAB), lactose transport and metabolism can be mediated by several pathways. Lactose-specific phosphoenol pyruvate-dependent phosphotransferase system (PTS) and P-b-galactosidase (P-b-gal) has been described in the genera Staphylococcus, Streptococcus, Lactococcus and Lactobacillus (de Vos & Vaughan, 1994). In Lactobacillus casei, lactose genes are transcribed as an operon, lacTEGF, encoding an antiterminator (LacT), lactose-specific PTS proteins (LacE and LacF) and a phospho-b-galactosidase (LacG) (Alpert & Chassy, 1988; Porter & Chassy, 1988; Alpert & Chassy, 1990; Alpert & Siebers, 1997; Gosalbes et al., 1997). In this microorganism, the lac operon is subject to induction by lactose through a mechanism of transcriptional antitermination in which LacT activity is negatively regulated by the lactose-specific enzymes, EIILac (Gosalbes et al., 1999). Moreover, the expression of this operon is repressed by glucose and other rapidly metabolizable carbon sources, partly through CcpA-mediated carbon catabolite repression (CCR) (Monedero et al., 1997; Viana et al., 2000). However, it has been shown that LacT and PTS elements are involved in an additional CcpA-independent CCR mechanism (Gosalbes et al., 1999).
Antitermination activity has been extensively studied in homologous proteins, such as BglG from E. coli (Mahadevan & Wright, 1987; Mahadevan et al., 1987; Schnetz et al., 1987; Schnetz & Rak, 1988, 1990; Amster-Choder et al., 1989; Houman et al., 1990; Amster-Choder & Wright, 1993; Görke & Rak, 1999) and SacT, SacY, LicT and GlcT in Bacillus subtilis (Zukowski et al., 1990; Crutz et al., 1990; Débarbouillé et al., 1990; Arnaud et al., 1992; Crutz & Steinmetz, 1992; Le Coq et al., 1995; Arnaud et al., 1996; Krüger & Hecker, 1996; Schnetz et al., 1996; Rutberg, 1997; Stülke et al., 1997; Tortosa et al., 1997; Bachem & Stülke, 1998; Idelson & Amster-Choder, 1998). In LAB, only two other antiterminator proteins have been reported: BglR in Lactococcus lactis and, recently, BglG from Lactobacillus plantarum (Bardowski et al., 1994; Marasco et al., 2000). Antitermination activity requires binding of these proteins to a ribonucleic antiterminator (RAT) sequence in the mRNA, stabilizing a stem-loop structure that prevents the formation of the transcriptional terminator located in the leader fragment preceding the coding regions (Aymerich & Steinmetz, 1992). The antiterminators show a modular organization with a RNA-binding region and two conserved domains, PRD (PTS Regulation Domain) (PRD-I and PRD-II), which, as demonstrated for BglG, LicT, SacT and SacY, contain phosphorylatable histidyl residues (two in each domain) that render them susceptible of regulation by PTS elements (Stülke et al., 1998; Tortosa et al., 1997). It has been proposed that PRD-I is negatively regulated by the sugar-specific PTS elements, EII, and PRD-II could be subject to a positive control by the general PTS protein, HPr (Stülke et al., 1998). Biochemical and genetic studies allowed to identify the phosphorylation sites in different regulators. However, despite the fact that they are well conserved, their regulatory role differs among them. The antiterminators SacT, GlcT and LicT from Bacillus subtilis are negatively controlled by EII-dependent phosphorylation, of one histidyl residue in PRD-I (His-97, His-104 and His-100, respectively) (Arnaud et al., 1992; Le Coq et al., 1995; Arnaud et al., 1996; Bachem & Stülke, 1998). However, in the PRD-containing activators LicR and LevR, of B. subtilis, and MtlR of B. stearothermophilus, the phosphorylation sites involved in this regulatory mechanism are located in the EIIA-like domain and PRD-II (Martin-Verstraete et al., 1998; Tobisch et al., 1999; Henstra et al., 2000). In addition, some of these regulators (SacT, LicT and LevR) are activated by HPr-dependent phosphorylation at different sites (Martin-Verstraete et al., 1995; Arnaud et al., 1996; Martin-Verstraete et al., 1998; Lindner et al., 1999). In Escherichia coli, the antiterminator BglG regulates the expression of bgl operon involved in b-glucoside transport. EIIBgl transports and phosphorylates b-glucosides but it also modulates BglG activity by phosphorylation. The His-208 of this antiterminator, located in PRD-II, was identified as the phosphorylation site (Chen et al., 1997; Chen et al., 2000). On the other hand, it has been recently shown that HPr also phosphorylates BglG, but the presumptive sites remain unidentified (Görke & Rak, 1999).
The aim of this work was to investigate the role of the conserved histidyl residues (His-101, His-159, His-210 and His-273) of LacT in the mechanisms that control antiterminator activity in L. casei. For this purpose, the potential phosphorylation sites of LacT have been replaced by alanine or aspartate by site-directed mutagenesis. The effect of these mutations on the expression of the lac operon has been evaluated through the determination of P-b-galactosidase activity from cultures grown under different induction/repression conditions.
METHODS:
Plasmids, bacterial strains and growth conditions.
L. casei strains and plasmids used in this work are listed in Table 1. L. casei cells were grown in MRS medium (Oxoid) and MRS fermentation broth (Adsa-Micro) plus 0.5% (w/v)of the different carbohydrates at 37 °C and 30 ºC under static conditions. E. coli DH5a was grown with shaking at 37 °C in Luria-Bertani (LB) medium. Plating of bacteria was performed on the respective media solidified with 1.5% agar. When required, the concentration of antibiotics used were 100 mg of ampicillin, 300 mg of erythromycin per ml to select E. coli transformants and 5 mg of erythromycin per ml for L. casei.
Table 1: Lactobacillus casei strains and plasmids used in this study.
| Strain or plasmid |
Description |
Source or reference |
| L. casei | ||
| CECT*5275 | wild type | B. Chassy |
| PL20 | CECT 5275 [pGAL9] | This work |
| BL195 | DlacT | This work |
| BL190 | DccpA | C.D. Esteban |
| Plasmids | ||
| pRV300 | Err | Leloup et al. (1997) |
| pRVDlacT | pRV300 with two fragments spanning the regions downstream and upstream of lacT | This work |
| pUC18 | Amr | Amersham Pharmacia Biotech |
| pLacT | pUC18 with lacT | This work |
| pLacTH101A | pLacT derivative (codon 101 of lacT is GCT for Ala) | This work |
| pLacTH101D | pLacT derivative (codon 101 of lacT is GAT for Asp) | This work |
| pLacTH159A | pLacT derivative (codon 159 of lacT is GCC for Ala) | This work |
| pLacTH210A | pLacT derivative (codon 210 of lacT is GCT for Ala) | This work |
| pLacTH210D | pLacT derivative (codon 210 of lacT is GAT for Asp) | This work |
| pLacTH273A | pLacT derivative (codon 273 of lacT is GCC for Ala) | This work |
| pGAL9 | Err | Pérez-Martínez et al. (1992) |
| pGALT | pGAL9 derivative expressing LacT | This work |
| pGALTH101A | pGAL9 derivative expressing LacTH101A | This work |
| pGALTH101D | pGAL9 derivative expressing LacTH101D | This work |
| pGALTH159A | pGAL9 derivative expressing LacTH159A | This work |
| pGALTH210A | pGAL9 derivative expressing LacTH210A | This work |
| pGALTH210D | pGAL9 derivative expressing LacTH210D | This work |
| pGALTH273A | pGAL9 derivative expressing LacTH273A | This work |
* CECT, Colección Española de Cultivos Tipo.
Recombinant DNA procedures.
Genomic DNA from Lactobacillus casei was purified using Purogene, DNA isolation Kit (Gentra Systems), following the procedure described by the manufacturer. Restriction and modifying enzymes were purchased commercially and used according to the recommendations of manufacturers. General cloning procedures were performed as described by Sambrook et al. (1989). L. casei strains were transformed by electroporation with a Gene-pulser apparatus (Bio-Rad Laboratories) as described elsewhere (Posno et al., 1991). PCR was performed using Expand TM High fidelity PCR system (Roche Molecular Biochemicals), containing 200 mM of each deoxynucleoside triphosphate and 10 pmol of each primer. Upon agarose gel electrophoresis, the amplified DNA was recovered with GFX TM PCR Kit (Amersham Pharmacia Biotech).
Construction of DlacT mutant.
Deletion of lacT was obtained by Campbell-like recombination using the integrative plasmid pRV300 (Leloup et al., 1997). Two fragments of 500 and 543 base pairs spanning the regions upstream and downstream of lacT, respectively, were obtained by PCR using total DNA of L. casei as template. The primers used were lac50 (5′-GAGCGGTACCATCAGTAGA-3′) and lac51 (5′-GTTGTCATCCCCTCCCAG-3′) for one fragment and lac52 (5′-CGCGCTCGCAGTCGTAGA-3′) and lac25 (5′-CGATATGAGCTCAGATC-3′) for the other fragment. The two PCR products were ligated and the ligation mix used as template in a PCR with lac50 and lac25. These two oligonucleotides carried KpnI and SacI sites (underlined), respectively, allowing the cloning of the last PCR product into KpnI/SacI-digested pRV300, giving pRVDlacT. This plasmid was used to transform L. casei. A transformant Lac+ and Er+ was grown for two hundred generations without antibiotic in order to allow the second recombination event, which would excise of the vector rendering Lac– Er– colonies. The proper first and second recombination events were confirmed by PCR. One of the lacT mutants was selected and the strain named BL195. Thus, in this strain lacT gene was totally deleted, while keeping the lac promoter, including all the initiation signals and regulatory sites unaltered upstream of the remaining genes of the lac operon.
Plasmid constructions and site-directed mutagenesis.
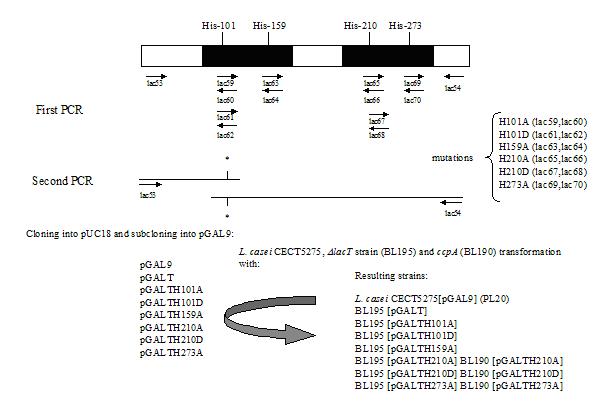
To obtain the plasmid pLacT, lacT was amplified with primers lac53 (5’-GCACTGGGAGGGGATGACAA-3’) and lac54 (5’-TTGGAAGCTTGCTTCAAAGCC-3’) using L. casei DNA as template, and the PCR product was cloned into the SmaI site of pUC18 using Sureclone kit (Amersham Pharmacia Biotech). Lac54 primer showed two substitutions (boldface) to introduce a new HindIII site (underlined). pLacT was used as template in the site-directed mutagenesis to replace the triplets encoding histidyl residues of LacT by alanine or aspartate (H101A, H101D, H159A, H210A, H210D and H273A). These amino acids (Ala and Asp) mimick non-phosphorylated and phosphorylated forms of the residue. The overlap extension method applied allows to introduce site-specific mutations and to generate a mutant gene in two PCR-steps (Ho et al., 1989). In the first PCR-step, two fragments of lacT were independently amplified using outside primers (lac53 or lac54) and the appropiate oligonucleotide of the following mutagenic primers (lac59 5′-TGAGTGATGCTATTTACGA and lac60 5′-TCGTAAATAGCATCACTCA, lac61 5′-TGAGTGATGATATTTACGA and lac62 5′-TCGTAAATATCATCACTCA, lac63 5′-TGGCGTTGGCCTTTATCAA and lac64 5′-TTGATAAAGGCCAACGCCA, lac65 5′-TTATGATTGCTCTCCAGTA and lac66 5′-TACTGGAGAGCAATCATAA, lac67 5′-TTATGATTGATCTCCAGTA and lac68 5′-TACTGGAGATCAATCATAA, lac69 5′-TCATCATCGCCATTCAGCG and lac70 5′-CGCTGAATGGCGATGATGA) (Fig.1). The mutated triplets are on boldface. These pairs of primers are complementary, hence, the strands of the fragments obtained overlaped and they could be extended in a subsequent reaction using lac53 and lac54 primers. The products of these second PCRs were cloned into pUC18/SmaI. The resulting plasmids were named pLacH101A, pLacH101D, pLacH159A, pLacH210A, pLacH210D (Fig.1). To replace His-273 by Ala residue lac2 (5’-CAACGATATAAGCGCAGATC) was used as external primer. The strategy was followed as described above and the plasmid obtained was designed as pLacH273A (Fig. 1). The orientation of the inserts in pUC18 vector was checked by digestion with the endonuclease HindIII. The fragments were subsequently sequenced to exclude PCR artifacts using a Perkin-Elmer Abi Prism 310 automated sequencer. Plasmids carrying wild type and mutated genes were digested with BamHI and HindIII and the resulting fragment cloned into BamHI/HindIII digested pGAL9 (Pérez-Martínez et al., 1992), generating pGALT, pGALTH101A, pGALTH101D, pGALTH159A, pGALTH210A, pGALTH210D, pGALTH273A. In these pGAL9-derived plasmids lacT variants are under control of the constitutive SPO2/AL9 promoter. These plasmids were used to transform L. casei CECT5275, DlacT strain (BL195) and ccpA mutant (Esteban C.D., unpublished data) (Fig. 1). L. casei CECT5275 [pGAL9] transformant was named PL20.
Enzymatic assay.
Phospho-b-galactosidase activity was assayed as previously described (Chassy et al., 1983). L. casei cells were permeabilized vortexing 5 min with 10 mM deoxicolic acid (Taranto, 1999).
RESULTS:
Effect of mutations in His-101 and His-159 on LacT activity.
LacT activity is negatively regulated by EIILac, as L. casei mutants in these elements displayed an inducer-independent antiterminator activity (Gosalbes et al., 1999). The conserved histidyl residues of PRD-I were replaced by Ala (H101A and H159A) and by Asp (H101D) to study their role in the induction mechanism of the lac operon. These lacT mutant genes were cloned under a constitutive promoter SPO2/AL9 in pGAL9 (Table 1 and Fig.1) and were used to complement a mutant with a chromosomal deletion of lacT (BL195), obtained by Campbell-like recombination. This DlacT mutant could not grown on lactose, since it was lacking the antiterminator. Then, BL195 was transformed with pGALT, pGALTH101A, pGALTH101D and pGALTH159A, and its complementation was initially tested on MRS fermentation plates with lactose. While BL195[pGALT], expressing wild type lacT from SPO2/AL9 promoter, and wild type strain (PL20) fermented this sugar, the same strain transformed with plasmids bearing mutated lacT (BL195[pGALTH101A], BL195[pGALTH101D] and BL195[pGALTH159A]) did not utilize lactose at 37 ºC. However, surprisingly, when grown at 30 ºC BL195[pGALTH101A] and BL195[pGALTH159A] showed a lactose+ phenotype. In order to confirm this fact, DlacT transformants carrying one of these two mutations (H101A or H159A) were grown at 37 ºC and 30 ºC on MRS fermentation broth supplemented with lactose and the P-b-gal activity was determined, as measure of the lac operon expression. Neither BL195[pGALTH101A] nor BL195[pGALTH159A] showed activity when grown at 37 ºC (4.66 ± 0.30 and 1.18 ± 0.64 nmol min-1mg-1dry weight, respectively), but a significant activity was detected at 30 ºC (22.55 ± 3.37 and 23.45 ± 5.48 nmol min-1mg-1dry weight, respectively).
The involvement of His-101 and His-159 in the induction mechanism of lac operon was studied in vivo by determining P-b-gal activity in BL195[pGALT], BL195[pGALTH101A], BL195[pGALTH101D], BL195[pGALTH159A] and wild type strains, when grown on ribose (non-inducing sugar), lactose (inducer) and glucose plus lactose (CCR) (Table 2). In BL195[pGALT], the expression of lac operon was restored on lactose grown cells, although P-b-gal activity was lower than that found in the wild type strain (PL20). Both strains, BL195[pGALT] and PL20, showed the same pattern of P-b-gal expression: induction by lactose and repression by glucose. This suggested that PRD-I from LacT could be dephosphorylated by EIILac even when it is overexpressed. In the absence of lactose (ribose grown cells), transformants with Ala replacements (BL195[pGALTH101A] and BL195[pGALTH159A]) showed about half the activity detected on lactose, suggesting an additive effect of these mutations. Alternatively, if each of the Ala replacements in PRD-I could render a fully active LacT, the structural instability provoked by these mutations may be the cause for the reduced activity detected. In contrast, no activity was detected in BL195[pGALTH101D] grown on any of the three sugars used (Table 2), indicating that the phosphorylation of this residue may inactivate LacT, which would complement the results obtained with the Ala replacements.
Table 2: P-b-galactosidase activity from different strains.
|
P-b-galactosidase* |
|||
| Strain |
Sugar |
||
|
Lactose |
Glucose + Lactose |
Ribose |
|
PL20 |
36 ± 2.56 |
ND |
0.84 ± 0.43 |
| BL195[pGAL9] |
†ND |
ND |
1.29 ± 0.15 |
| BL195[pGALT] |
19.34 ± 5.86 |
2.02 ± 0.73 |
1.88 ± 0.25 |
| BL195[pGALTH101A] |
22.55 ± 3.37 |
1.20 ± 0.44 |
13.07 ± 4.02 |
| BL195[pGALTH101D] |
ND |
1.40 ± 0.66 |
1.34 ± 0.60 |
| BL195[pGALTH159A] |
23.45 ± 5.48 |
1.94 ± 0.45 |
12.35 ± 3.70 |
| BL195[pGALTH210A] |
ND |
2.3 ± 0.34 |
2.15 ± 1.06 |
| BL195[pGALTH210D] |
ND |
1.66 ± 0.70 |
0.74 ± 0.22 |
| BL195[pGALTH273A] |
ND |
0.60 ± 0.13 |
0.97 ± 0.32 |
| BL190[pGAL9] |
44.60 ± 3.65 |
ND |
ND |
| BL190[pGALT] |
44.86 ± 3.22 |
40.90 ± 0.58 |
ND |
| BL190[pGALTH210A] |
49.49 ± 2.5 |
ND |
ND |
| BL190[pGALTH210D] |
46.63 ± 1.45 |
16.26 ± 0.96 |
ND |
| BL190[pGALTH273A] |
48.42 ± 3.25 |
ND |
ND |
* P-b-galactosidase activity is expressed as nanomoles of o-nitrophenyl-b-D-galactopyranoside-6-phosphate hydrolysed per minute and milligram of dry weight. The values and standard deviations are from at least three independent experiments. P-b-gal activity from BL195[pGALTH101A] and BL195[pGALTH159A] corresponds to the cultures grown at 30 ºC.
† ND, Not detected
Characterization of putative phosphorylation sites in the PRD-II of LacT.
Sequence alignment of proteins belonging to BglG family led to select His-210 and His-273 of LacT as potential phosphorylation sites. Thus, strains (BL195[pGALTH210A], BL195[pGALTH210D] and BL195[pGALTH273A]) were constructed with the histidyl residues (His-210 and His-273) replaced by Ala or Asp, mimicking non-phosphorylated and phosphorylated forms, respectively. In this case, thermosensitivity was also tested on MRS fermentation plates supplemented with lactose at 37 ºC and 30 ºC. None of the mutants grew on lactose at either temperature used. In fact, no P-b-gal activity was detected on ribose- or glucose + lactose-grown cells in these mutants (Table 2).
To study the involvement of PRD-II (His-210 and His-273) in CCR, a DccpA mutant (BL190) was transformed with pGAL9, pGALT, pGALTH210A, pGALTH210D and pGALTH273A. All the transformants showed P-b-galactosidase activity on lactose-grown cells due to the normal expression of the chromosomal lac operon in the host strain BL190. However, any effect on the expression of the lac operon in BL190 on glucose + lactose medium, would be exclusively due to LacT-mediated CCR. Expression of LacT from a multicopy plasmid (pGALT) in ccpA mutant conferred glucose insensitivity, possibly due to dephosphorylation of PRD-II when the inducer was present and in conditions of LacT molar excess. Expression of the mutation H210D in BL190 resulted in the activation of the operon in the presence of glucose and the inducer, lactose, indicating that this mutation was partially active and insensitive to glucose repression in the ccpA mutant. However, Ala replacements did not render an active form of antiterminator protein, since the repressing effect of glucose on P-b-gal activity remained in BL190[pGALH210A] and BL190[pGALH273A] (Table 2).
DISCUSSION:
The antiterminator LacT regulates lactose operon (lacTEGF) expression in Lactobacillus casei. The activity of this protein is controlled by EIILac and PTS elements in response to the availability of lactose or the presence of glucose (Gosalbes et al., 1999; Monedero et al., 1997; Viana et al., 2000). Sequence analysis of the members of BglG family revealed a modular organization containing a RNA binding domain and PTS-regulated domains (PRD-I and PRD-II) with usually four well-conserved potential phosphorylation sites (two in each domain). PRD-containing regulators could be subject to a dual regulation by phosphorylation: negative control by EII exerted on PRD-I and positive control by HPr-His-P generally on PRD-II. Then, His-101, His-159 in PRD-I and His-210, His-273 in PRD-II of LacT were mutated to investigate their role in the phosphorylation-dependent regulatory mechanisms.
BL195 (DlacT) carried a complete deletion of lacT and, therefore, it had Lac– phenotype. The operator, RAT sequence and terminator structure, remained functional, as demonstrated by the fact that constitutive expression of LacT from pGALT partially restored growth on lactose and showed the same expression pattern as the wild type, glucose repression and induction by lactose. However, other works reporting overproduction of antiterminators, such as LicT, substrate induction was eliminated (Krüger & Hecker, 1995; Krüger et al., 1996).
Antiterminator activity of all members of the BglG family of antiterminators has been shown to be subject to substrate induction through phosphate exchange with the sugar-specific EII PTS elements (Stülke et al., 1998). In L. casei, transcriptional studies of the lac operon in lacE and lacF mutants showed an inducer-independent antiterminator activity, indicating a negative control of these elements (Gosalbes et al., 1999). According to the model of PTS-mediated regulation of antiterminators (Stülke et al., 1998), EIILac would dephosphorylate LacT PRD-I in the presence of lactose to yield its active form. EII-dependent negative control has been demonstrated to involve, at least, one of two conserved histidines of PRD-I in LicT, SacY, SacT and GlcT (His-101, His-99, His-97 and His-104, respectively) (Schnetz et al., 1987; Débarbouillé et al., 1990; Arnaud et al., 1992; Arnaud et al., 1996; Stülke et al., 1997; Bachem & Stülke, 1998). PRD-I alignment of different antiterminators located highly conserved histidines at positions His-101 and His-159 in LacT. In this work, the replacement of His-101 or His-159 by Ala rendered LacT active in absence of lactose (ribose grown cells), although the P-b-gal activity observed in these strains (BL195[pGALTH101A] and BL195[pGALTH159A]) was lower than that detected on lactose grown cells. It could be proposed that both residues were involved in the lactose induction process and one or both of them should be dephosphorylated to obtain full LacT activity. These replacements of His-101 and His-159 by Ala yielded the same phenotype of thermosensitive LacT, suggesting that these histidyl residues may interact and be playing a role in the conformational stability of PRD-I in LacT, as it has recently proposed from the resolved structure of PRDs in LicT (van Tilbeurgh et al., 2001).
LacT activity has also been shown to be sensitive to the presence of glucose, which caused the already reported CcpA-independent CCR of L. casei lac operon (Monedero et al., 1997; Gosalbes et al., 1999; Viana et al., 2000). In homologous antiterminators, this event apparently required phosphorylation of PRD-II by HPr-His-P, although this effect varied slightly among them. While SacT and LicT are subject to a positive control by HPr-dependent phosphorylation, SacY and GlcT activities do not require it. LicT can be phosphorylated by HPr at three sites, one in PRD-I (His-159) and the other two in PRD-II (His-207 and His-269). Furthermore, His-207 is preferentially phosphorylated indicating a fine modulation of LicT activity (Lindner et al., 1999). In contrast, HPr-dependent phosphorylation at the equivalent sites (His-207 and His-269) in SacY does not affect its activity (Tortosa et al., 1997). The total loss of activity in BL195[pGALTH210A] and BL195[pGALTH273A], grown on lactose, suggested that both His residues (His-210 and His-273) should be phosphorylated to obtain the active form of LacT. All the mutants and wild type showed a remarkable glucose repression effect, as expression of the lac operon is also by CcpA-dependent CCR in this genetic background (DlacT). Thus, we studied the involment of His-210 and His-273 in LacT-mediated CCR using a ccpA mutant. Strikingly, a derepressed phenotype was observed in BL190[pGALT], possibly due to the overexpression of LacT from a constitutive promoter in a high copy number plasmid. Also, a likely expression of the lac operon was found when mutant H210D was expressed in a ccpA background, although activity was lower than expected if the mutant was fully activated or insensitive to the glucose effect, as observed in LicT (van Tilbeurgh et al., 2001). Although this mutation was not temperature-sensitive, it may be introducing conformational changes or difficulties in dimer formation, which is apparently required for activity in E.coli BglG (Amster-Choder & Wright, 1992, 1993; Boss et al. 1999). In E. coli it has recently been proposed that HPr-dependent phosphorylation of PRD-II is necessary for dimer formation, the active form of BglG (Görke & Rak, 1999) and a similar model was initially suggested for LicT (Krüger & Hecker, 1995; Krüger et al., 1996). However, recent data obtained from the crystallized regulatory domains of LicT (van Tilbeurgh et al., 2001) showed that phosphorylated histidines in PRDs are facing inwards, hidden from interactions with the paired molecule in the dimer. Hence, the negative charge introduced in LacT(H210D) might be introducing structural changes that indirectly alter the dimer interface. In BL190[pGALTH210D], the overproduced LacT(H210D) may be forming heterodimers with the chromosomally encoded LacT with weak antiterminator activity. In contrast, in BL195 (DlacT) only an unstable homodimer would be formed from pGALTH210D. Nevertheless, the role of the PRD-II histidines in glucose inactivation of LacT has been confirmed with the H210A and H273A mutants as they lack of activity on any background.
Undoubtedly, further studies are required to clarify the mechanism by which phosphorylation regulates dimerization and activity of LacT. Furthermore, in vitro phosphorylation experiments with the different purified forms of LacT will be necessary to confirm the proposed phosphorylation processes.
In conclusion, this work described the in vivo effect of site-directed mutations of LacT antiterminator on lac operon expression from Lactobacillus casei. Dephosphorylated PRD-I histidyl residues, His-101 and His-159, are indispensable for full induction of lac operon, whilst His-210 and His-273 of PRD-II play a role in CcpA-independent carbon catabolite repression. Results obtained are suggesting that there could be differences in the affinity of different phospho-transfer elements for PRD-I and PRD-II histidines.
ACKNOWLEDGMENTS:
We thank D. Coloma for her technical assistance with the automated sequencer. C.D.E. was the recipient of a fellowhip from Spanish government. This work was financed by the EU project BIO4-CT96-0380 and by funds of the Spanish CICyT (Interministerial Commision for Science and Technology) (Ref. ALI 98-0714).
REFERENCES:
Albert, T. (1989). Mutational effects on protein stability. Annu Rev Biochem 58:765-798.
Alpert, C.-A. & Chassy, B. M. (1988). Molecular cloning and nucleotide sequence of the factor IIIlac gene of Lactobacillus casei. Gene 62:277-288.
Alpert, C.-A. & Chassy, B. M. (1990). Molecular cloning and DNA sequence of lacE, the gene encoding the lactose-specific enzyme II of the phosphotransferase system of Lactobacillus casei. J Biol Chem 265:22561-22568.
Alpert, C.-A., & Siebers, U. (1997). The lac operon of Lactobacillus casei contains lacT, a gene coding for a protein of BglG family of transcriptional antiterminators. J Bacteriol 179:1555-1562.
Amster-Choder, O., & Wright, A. (1992). Modulation of dimerization of a transcriptional antiterminator protein by phosphorylation. Science 257:1395-1397.
Amster-Choder, O., & Wright, A. (1993). Transcriptional regulation of bgl operon of Escherichia coli involves phosphotransferase system-mediated phosphorylation of a transcriptional antiterminator. J Cell Biochem 51:83-90.
Amster-Choder, O., Houman, F., & Wright, A. (1989). Protein phosphorylation regulates transcription of the b-glucoside utilization operon in E. coli. Cell 58:847-855.
Arnaud, M., Vary, P., Zagorec, M., Klier, A., Débarbouillé, M., Postma, P., & Rapoport, G. (1992). Regulation of the sacPA operon of Bacillus subtilis: Identification of phosphotransferase system components involved in SacT activity. J Bacteriol 174:3161-3170.
Arnaud, M., Débarbouillé, M., Rapoport, G., Saier, M. H. Jr., & Reizer, J. (1996). In vitro reconstitution of transcriptional antiterminator by the SacT and SacY proteins of Bacillus subtilis. J Biol Chem 271:18966-18972.
Aymerich, S. & Steinmetz, M. (1992). Specificity determinants and structural features in the RNA target of the bacterial antiterminator proteins of the BglG/SacY family. Proc Natl Acad Sci USA 89:10410-10414.
Bachem, S. & Stülke, J. (1998). Regulation of the Bacillus subtilis GlcT antiterminator protein by components of phosphotransferase system. J Bacteriol 180:5319-5326.
Bardowski, J., Ehrlich, S. D., & Chopin, A. (1994). BglR protein, which belongs to the BglG family of transcriptional antiterminators, is involved in b-glucoside utilization in Lactococcus lactis. J Bacteriol 176:5681-5685.
Boss, A., Nussbaum-Shochat, A., & Amster-Choder O. (1997). Characterization of the dimerization domain in BglG, an RNA-binding transcriptional antiterminator from Escherichia coli. J Bacteriol 181:1755-1766.
Chassy, B. M. & Thompson, J. (1983). Regulation of lactose-phosphoenolpyruvate-dependent-phosphotransferase system and b-D-phosphogalactosidase galactohydrolase activities in Lactobacillus casei. J Bacteriol 154: 1195-1203.
Chen, Q., Engelberg-Kulka, H. & Amster-Choder, O. (1997). The localization of the phosphorylation site of BglG, the response regulator of the Escherihia coli bgl sensory system. J Biol Chem 272: 17263-17268.
Chen, Q., Postma, P. W. & Amster-Choder, O. (2000). Dephosphorylation of Escherihia coli transcriptional antiterminator BglG by the sugar sensor BglF is the reversal of its phosphorylation. J Bacteriol 182:2033-2036.
Crutz, A. M., & Steinmetz, M. (1992). Transcription of the Bacillus subtilis sacX and sacY genes, encoding regulators of sucrose metabolism, is both inducible by sucrose and controlled by the DegS-DegU signalling system. J Bacteriol 174:6087-6095.
Crutz, A. M., Steinmetz, M., Aymerich, S., Richter, R. & Le Coq, D. (1990). Induction of levansucrase in Bacillus subtilis: an antitermination mechanism negatively controlled by the phosphotransferase system. J Bacteriol 172: 1043-1050.
Débarbouillé, M., Arnaud, M., Fouet, A., Klier, A. & Rapoport, G. (1990). The sacT gene regulating the sacPA operon in Bacillus subtilis shares strong homology with transcriptional antiterminators. J Bacteriol 172: 3966-3973.
de Vos, W. M., & Vaughan, E. E. (1994). Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol Rev 15: 217-237.
Görke, B. & Rak, B. (1999). Catabolite control of Escherichia coli regulatory protein BglG activity by antagonistically acting phosphorylations. EMBO J 18:3370-3379.
Gosalbes, M. J., Monedero, V., Alpert, C.-A., & Pérez-Martínez, G. (1997). Establishing a model to study the regulation of the lactose operon in Lactobacillus casei. FEMS Microbiol Lett 148: 83-89.
Gosalbes, M. J., Monedero, V. & Pérez-Martínez, G. (1999). Elements involved in catabolite repression and substrate induction of the lactose operon in Lactobacillus casei. J Bacteriol 181:3928-3934.
Henstra, S.A., Duurkens, R.H. & Robillard, G.T. (2000). Multiple phosphorylation events regulate the activity of the mannitol transcriptional regulator MtlR of the Bacillus stearothermophilus phosphoenolpyruvate-dependent mannitol phosphotransferase system. J. Biol. Chem. 10: 7037-7044.
Ho, S. N., Hunt, H. D., Horton, R. M., Pullen, J. K. & Pease, L. R. (1989). Site-directed mutagenesis by overlap extension using polymerase chain reaction. Gene 71:51-59.
Houman, F., Diaz-Torres, M. R., & Wright, A. (1990). Transcriptional antitermination in bgl operon of E. coli is modulated by a specific RNA binding protein. Cell 62:1153-1163.
Idelson, M., & Amster-Choder, O. (1998). SacY, a transcriptional antiterminator from Bacillus subtilis, is regulated by phosphorylation in vivo. J Bacteriol 180:660-666.
Krüger, S., & Hecker, M. (1995). Regulation of the putative bglPH operon for aryl-b-glucoside utilization in Bacillus subtilis. J Bacteriol 177: 5590-5597.
Krüger, S., Gertz, S., & Hecker, M. (1996). Transcriptional analysis of bglPH expression in Bacillus subtilis: Evidence for two distinct pathways mediating carbon catabolite repression. J Bacteriol 178:2637-2644.
Le Coq, D., Lindner, C., Krüger, S., Steinmetz, M., & Stülke, J. (1995). New b-glucoside (bgl) genes in Bacillus subtilis: the bglP gene product has both transport and regulatory functions similar to those of BglF, its Escherichia coli homolog. J Bacteriol 177:1527-1535.
Leloup, L., Ehrlich, S. D., Zagorec, M. & Morel-Deville, F. (1997). Single crossing-over integration in the Lactobacillus sake chromosome and insertional inactivation of the pts and the lacI genes. Appl Environ Microbiol 63: 2117-2123.
Lindner, C., Galinier, A., Hecker, M. & Deutscher, J. (1999). Regulation of the activity of the Bacillus subtilis antiterminator LicT by multiple PEP-dependent, enzyme I- and HPr-catalysed phosphorylation. Mol Microbiol 31:995-1006.
Marasco, R., Salatiello, I., de Felipe, M. & Sacco, M. (2000). A physical and functional analysis of the newly-identified bglGPT operon of Lactobacillus plantarum. FEMS Microbiol Lett 186: 269-273.
Mahadevan, S., & Wright, A. (1987). A bacterial gene involved in transcription antitermination: regulation at a Rho-independent terminator in bgl operon of E. coli. Cell 50:485-494.
Mahadevan, S., Reynolds, A. E. & Wright, A. (1987). Positive and negative regulation of bgl operon in Escherichia coli. J Bacteriol 169:2570-2578.
Martin-Verstraete, I., Stülke, J., Klier, A. & Rapoport, G. (1995). Two different mechanisms mediate catabolite repression of the Bacillus subtilis levanase operon. J Bacteriol 177: 6919-6927.
Martin-Verstraete, I., Charrier V., Stülke, J., Galinier, A., Erni, B., Rapoport, G., & Deutscher, J. (1998). Antagonistic effects of dual PTS-catalysed phosphorylation on the Bacillus subtilis transcriptional activator LevR. Mol Microbiol 28:293-303.
Monedero, V., Gosalbes, M. J., & Pérez-Martínez, G. (1997). Catabolite repression in Lactobacillus casei ATCC 393 is mediated by CcpA. J Bacteriol 179: 6657-6664.
Pérez-Martínez, G., Kok, J., Venema, G, van Dijl, J. M., Smith, H. & Bron, S. (1992). Protein export elements from Lactococcus lactis. Mol Gen Genet 234:401-411.
Porter, E. V. & Chassy, B. M. (1988). Nucleotide sequence of the b-D-phospho-galactosidase gene of Lactobacillus casei: comparison to analogous pbg genes of other Gram-positive organisms. Gene 62:263-276.
Posno, M., Leer, R. J., van Luijk, N., van Giezen, M. J. F., Heuvelmans, P. T. H. M., Lokman, B. C. & Pouwels, P. H. (1991). Incompatibility of Lactobacillus vectors with replicons derived from small cryptic Lactobacillus plasmids and segregational instability of the introduced vectors. Appl Environ Microbiol 57:1822-1828.
Rutberg, B. (1997). Antitermination of transcription of catabolic operons. Mol Microbiol 23:413-421.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular Cloning: a Laboratory Manual, 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
Schnetz, K., & Rak, B. (1988). Regulation of bgl operon of Escherichia coli by transcriptional antitermination. EMBO J 7:3271-3277.
Schnetz, K., & Rak, B. (1990). b-Glucoside permease represses the bgl operon of E. coli by phosphorylation of the antiterminator protein and also interacts with glucose-specific enzyme II, the key element in catabolic control. Proc Natl Acad Sci USA 87: 5074-5078.
Schnetz, K., Toloczki, C., & Rak, B. (1987). b-Glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol 169:2579-2590.
Schnetz, K., Stülke, J., Gertz, S., Krüger, S., Krieg, M., Hecker, M. & Rak; B. (1996). LicT, a Bacillus subtilis transcriptional antiterminator protein of the BglG family. J Bacteriol 178: 1971-1979.
Stülke, J., Martin-Verstraete, I., Zagorec, M., Rose, M., Klier, A. & Rapoport, G. (1997). Induction of Bacillus subtilis ptsGHI operon by glucose is controlled by a novel antiterminator, GlcT. Mol Microbiol 25:65-78.
Stülke, J., Arnaud, M., Rapoport, G., & Martin-Verstraete, I. (1998). PRD-a protein domain involved in PTS-dependent induction and carbon catabolite repression of catabolic operons in bacteria. Mol Microbiol 28:865-874.
Taranto, M.P. (1999). Reducción de colesterol e hidrólisis de sales biliares por bacterias lácticas. PhD-thesis, Universidad Nacional de Tucumán, Argentina.
Tobisch, S., Stülke, J., & Hecker, M. (1999). Regulation of the lic operon of Bacillus subtilis and characterization of potencial phosphorylation sites of the LicR regulator protein by site-directed mutagenesis. J Bacteriol 181:4995-5003.
Tortosa, P., Aymerich, A., Lindner, C., Saier, M.H.Jr., Reizer, J & Le Coq, D. (1997). Multiple phosphorylation of SacY, a Bacillus subtilis antiterminator negatively controlled by the phosphotransferase system. J Biol Chem 272:17230-17237.
Van Tilbeurgh, H., Le Coq, D. & Declerck, N. (2001). Crystal structure of an activated form of the PTS regulation domain from the LicT transcriptional antiterminator. EMBO J 20: 3789-3799.
Veyrat, A., Monedero, V., & Pérez-Martínez, G. (1994). Glucose transport by the phosphoenolpyruvate: mannose phosphotransferase system in Lactobacillus casei ATCC 393 and its role in carbon catabolite repression. Microbiology 140:1141-1149.
Viana, R., Monedero, V., Dossonnet, V., Vadeboncoeur, Ch., Pérez-Martínez, G. & Deutscher, J. (2000). Enzyme I and HPr from Lactobacillus casei: their role in sugar transport, carbon catabolite repression and inducer exclusion. Mol Microbiol 36:570-584.
Zukowski, M., Miller, L., Cosgwell, P., Chen, K., Aymerich, A. & Steinmetz, M. (1990). Nucleotide sequence of the sacS locus of Bacillus subtilis reveals the presence of two regulatory genes. Gene 90:153-155.

